ISO 13485:2016 is an internationally recognized standard for quality management systems (QMS) in the medical device industry. Designed specifically to meet the stringent regulatory requirements of this sector, it ensures organizations consistently deliver safe and effective medical devices. Adopting this standard is crucial for companies aiming to maintain compliance, enhance customer satisfaction, and enter global markets.
In this article, we’ll explore the essentials of ISO 13485:2016, its key benefits, implementation steps, and practical insights to help your organization achieve certification.
What is ISO 13485:2016?
ISO 13485:2016 specifies requirements for a QMS in organizations involved in the design, production, installation, and servicing of medical devices. It aligns closely with regulatory requirements, emphasizing risk management, process control, and product traceability.
Unlike ISO 9001, which applies broadly to various industries, ISO 13485 is tailored to address the unique needs of the medical device sector. While not mandatory in every region, certification is often required by regulatory bodies, including the FDA in the United States and the European Union’s CE marking process.
Key Features of ISO 13485:2016
ISO 13485:2016 focuses on several critical aspects that differentiate it from other QMS standards:
- Risk Management: A robust framework for identifying and mitigating risks throughout the product lifecycle.
- Regulatory Compliance: Alignment with international and regional regulatory requirements.
- Product Safety and Effectiveness: Ensuring that medical devices meet intended performance criteria.
- Traceability: Comprehensive documentation for tracking materials, processes, and products.
- Supplier Control: Strengthened oversight of suppliers and third-party partners.
Benefits of ISO 13485:2016 Certification
- Regulatory Compliance
Certification demonstrates compliance with global regulations, facilitating access to international markets such as the EU, US, and Japan.
- Improved Product Quality
ISO 13485:2016 establishes rigorous quality standards, leading to safer and more effective medical devices.
- Enhanced Risk Management
The standard promotes a proactive approach to identifying and addressing risks, reducing product recalls and safety issues.
- Increased Customer Trust
Certification signals a commitment to quality and safety, enhancing trust among customers, regulators, and partners.
- Competitive Advantage
Certified organizations stand out in the highly competitive medical device industry, making it easier to attract clients and secure contracts.

Who Needs ISO 13485:2016?
ISO 13485:2016 is relevant to a wide range of organizations in the medical device industry, including:
- Manufacturers: Companies producing medical devices for end-users.
- Suppliers: Providers of components or materials used in device production.
- Distributors: Organizations handling the logistics and delivery of medical devices.
- Service Providers: Entities involved in device maintenance, installation, or calibration.
Key Requirements of ISO 13485:2016
ISO 13485:2016 includes several mandatory requirements, such as:
- QMS Documentation: Detailed quality manuals, procedures, and records.
- Management Responsibility: Top management’s commitment to quality objectives.
- Risk Management Processes: Systematic risk assessment and mitigation.
- Product Realization: Rigorous control over design, development, production, and delivery processes.
- Monitoring and Measurement: Regular audits, inspections, and performance evaluations.
Steps to Implement ISO 13485:2016
- Conduct a Gap Analysis
Evaluate your existing processes against the standard’s requirements to identify areas for improvement.
- Develop Documentation
Prepare a quality manual, procedures, and work instructions to meet ISO 13485:2016 standards.
- Establish Risk Management Practices
Implement a robust risk management framework to address potential issues in the product lifecycle.
- Train Employees
Educate your workforce about ISO 13485:2016 requirements and their role in achieving compliance.
- Monitor Suppliers
Develop a supplier management system to ensure compliance with quality standards.
- Conduct Internal Audits
Perform regular audits to identify non-conformities and take corrective actions.
- Certification Audit
Engage an accredited certification body to evaluate your QMS. Address any findings to achieve certification.
Practical Tips for ISO 13485:2016 Certification
- Engage Leadership
Secure top management’s support for the implementation process, as their commitment is critical to success.
- Focus on Training
Ensure employees understand their roles in maintaining compliance and contributing to quality objectives.
- Simplify Documentation
Avoid over-complicating your QMS documentation; focus on clarity and usability.
- Use Technology
Leverage QMS software to streamline documentation, risk management, and auditing processes.
- Collaborate with Experts
Consider working with consultants or training providers to navigate complex requirements.
Challenges in Implementing ISO 13485:2016
- Navigating Regulatory Requirements
Understanding and aligning with international regulations can be challenging. Stay updated on changes in compliance standards.
- Resource Constraints
Allocating sufficient time, personnel, and budget is essential for successful implementation.
- Supplier Compliance
Ensuring suppliers meet ISO 13485 standards requires thorough evaluation and ongoing monitoring.
Case Study: Successful ISO 13485:2016 Implementation
MediTech Solutions, a small medical device manufacturer, sought ISO 13485:2016 certification to expand into international markets. By streamlining their documentation, adopting a robust risk management process, and training employees, they achieved certification within six months. This milestone allowed them to secure contracts with global distributors and increase revenue by 40%.
Conclusion
ISO 13485:2016 is a vital standard for organizations in the medical device industry, ensuring the production of safe and effective products while meeting stringent regulatory requirements. Certification not only improves quality and risk management but also opens doors to global markets and builds trust among stakeholders.
By understanding the standard’s requirements, engaging leadership, and leveraging best practices, your organization can achieve ISO 13485:2016 certification and set itself apart in a competitive industry.
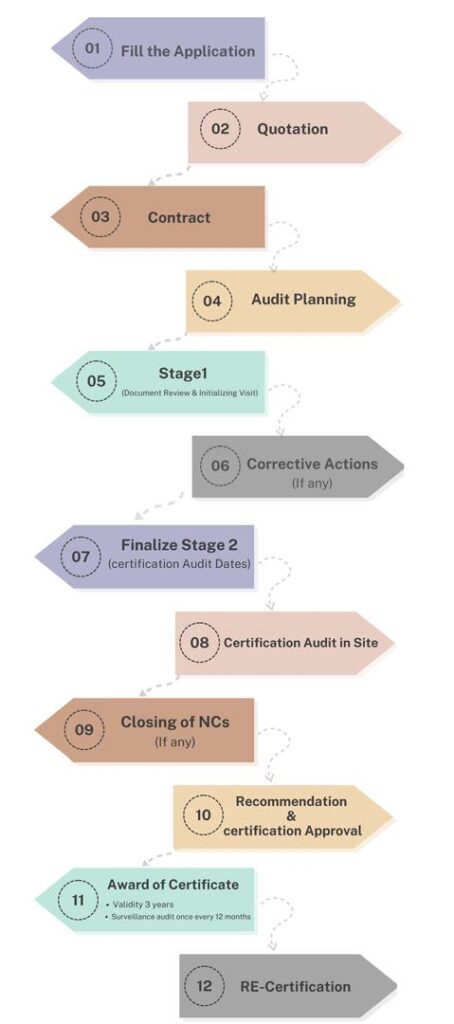
Certification Process
1- Completion of information related to the company name, company activities, the number of staff per work shift, website address, and branches.
2- Announcement of costs related to the requested certification issuance process.
3- Preparation and approval of the contract for implementation.
4- Planning the audit process and notifying the client for audit process.
5- reviewing documents to identify the client strengths and weaknesses in comparison to the established documentation.
6- Corrective actions for potential weaknesses.
7- Final planning for conducting the audit, registration, and certification issuance.
8- Conducting the on-site audit process at the client by the specialized team of the certifying company.
9- Corrective actions for potential weaknesses in the execution of the organization’s processes.
10- Recommendation for the issuance of a certification related to the client to the head office.
11- Achieving the related certification.
12- Renew after three years.